Modafinil: a Drug of Many Hats
Modafinil
Modafinil is a wakefulness promoting agent approved for medical use in the US. It is indicated for adult patients with excessive sleepiness that is associated with obstructive sleep apnea (OSA), narcolepsy, or shift work disorder (SWD).1 Modafinil along with its analogues are also currently being investigated for fatigue, substance addictions, psychiatric disorders, and neurological health benefits. Modafinil was approved as a medication in 1998. After more than 20 years Modafinil may be more popular and prevalent than ever. It appears you cannot listen to a podcast or read a popular article without the mention of Modafinil or one of its prodrugs or analogues. Even though it is a prescription medication it can be easily ordered via the “Clearnet” from international sources. Its prodrug, adrafinil, quite often pops its head into OTC supplements. Its analogues CRL-40,940 AKA Flmodafinil and its prodrug, CRL-40,941 AKA Fladrafinil are commonly available as research chemicals or nootropic powders. The more obscure drug, Modafiendz is the N-methylated derivative of Flmodafinil. It can be found on recreational drug oriented, research chemical sites.
I reference Modafinil as “The Drug of Many Hats” because there are so many proclaimed uses, names, and derivatives. Modafinil and its close relatives have been touted as many things; stimulant, eugeroic, limitless pill, real life NZT-48, Adderall replacement, cognitive enhancer, mindfulness pill, and smart drug among other things. There are brand names such as Nuvigil, Provigil, Movigil, Vigil, Modvigil, Modalert, Wakelert, and the list goes on! Modafinil is utilized by those with the conditions previously mentioned; it is also used off label by those seeking a performance edge, neurological enhancement, and/or neurological health benefit. Anecdotes of CEOs and widespread use throughout Silicon Valley are often boasted in articles. There have been worldwide military studies and approval for use of Modafinil in specific operations. The US Airforce no longer approves dextroamphetamine use but now approves the use of Modafinil, this is a real life application of its “Adderall alternative” title.2 Modafinil’s sustained popularity is due to its ability to promote cognitive enhancement, focus, motivation, and alertness with seemingly little side effects.
What is Modafinil?
Modafinil is the psychostimulant, Diphenylmethylsulfinylacetamide or 2-[(Diphenylmethyl)sulfinyl]acetamide, which acts primarily on the monoaminergic system. The mechanism of action for this compound is not completely understood. Modafinil seems to produce similar effects to classic central nervous system stimulants but via a slightly different mechanism. Modafinil seems to be less euphoric and less addictive than the classic CNS stimulants. Research makes it apparent that it acts in a unique manner when compared to amphetamines and phenidates. Despite its unique mode of action and decreased potential for abuse this excerpt directly from the Nuvigil insert suggests the possibility for habitual or recreational use is not completely absent:
“In addition to its wake-promoting effects and ability to increase locomotor activity in animals, modafinil produces psychoactive and euphoric effects, alterations in mood, perception, thinking, and feelings typical of other CNS stimulants in humans. Modafinil has reinforcing properties, as evidenced by its self-administration in monkeys previously trained to self-administer cocaine; modafinil was also partially discriminated as stimulant-like”
Modafinil Pharmacology
There is a considerable amount of information on the pharmacology of modafinil. The mechanisms of this drug are not completely understood. Therefore, I will include the information that I have identified as most conclusive and try to eliminate any material that needs further research or interpretation.
Modafinil exhibits a mild and selective affinity for the dopamine transporter (DAT).3,5 However, Wisor JP et al 2001 found that DAT knockout mice were “unresponsive to the normally robust wake-promoting action of modafinil,” this statement suggests that the DAT is an integral part of modafinils mode of action. Unlike amphetamine, modafinil does not cause dopamine release.4 It is considered a weak norepinephrine transporter inhibitor.6 Modafinil does not directly affect GABA but seems to decrease GABA via a serotonergic pathway. 7,8 Modafinil increases serotonin in specific areas of the brain but like its effect on GABA, serotonin seems to increase through indirect methods. 10,11 There does not seem to be any effect on serotonin reuptake. 12 Orexin (hypocretin) is a neuropeptide which is involved in arousal and wakefulness. Low production of orexin is the cause for of the most common form of narcolepsy. Modafinil has been shown to activate orexin containing neurons. 13 Interestingly, Willie JT et al 2005 found “the presence of orexin is not necessary for the wakefulness-prolonging action of modafinil.” There is evidence that suggests modafinil is neuroprotective, antiparkinsonian, and exhibits an antioxidative effect on the brain. 16,17 Modafinil is also a cognitive enhancer in some populations, this effect seems to need further research to produce more conclusive information.
Armodafinil vs Modafinil Pharmacokinetics
Modafinil exists as a racemic compound which means there are multiple isomers of the drug. Isomers are geometrically similar but not superimposable on each other. The two isomers are S-(+)-modafinil and R-(-)-modafinil (armodafinil). R has a threefold higher affinity for the DAT than the S enantiomer.18 R-modafinil is also metabolized at a rate that is threefold slower than S-modafinil, which means it will produce its effects for a longer period of time.19 These studies show us that the R enantiomer is stronger with a longer half-life. One may desire R-modafinil if they prefer longer and more pronounced effects while perhaps using S-modafinil if they prefer a shorter and milder effect.
Modafinil and CRL Prodrugs / Precursors
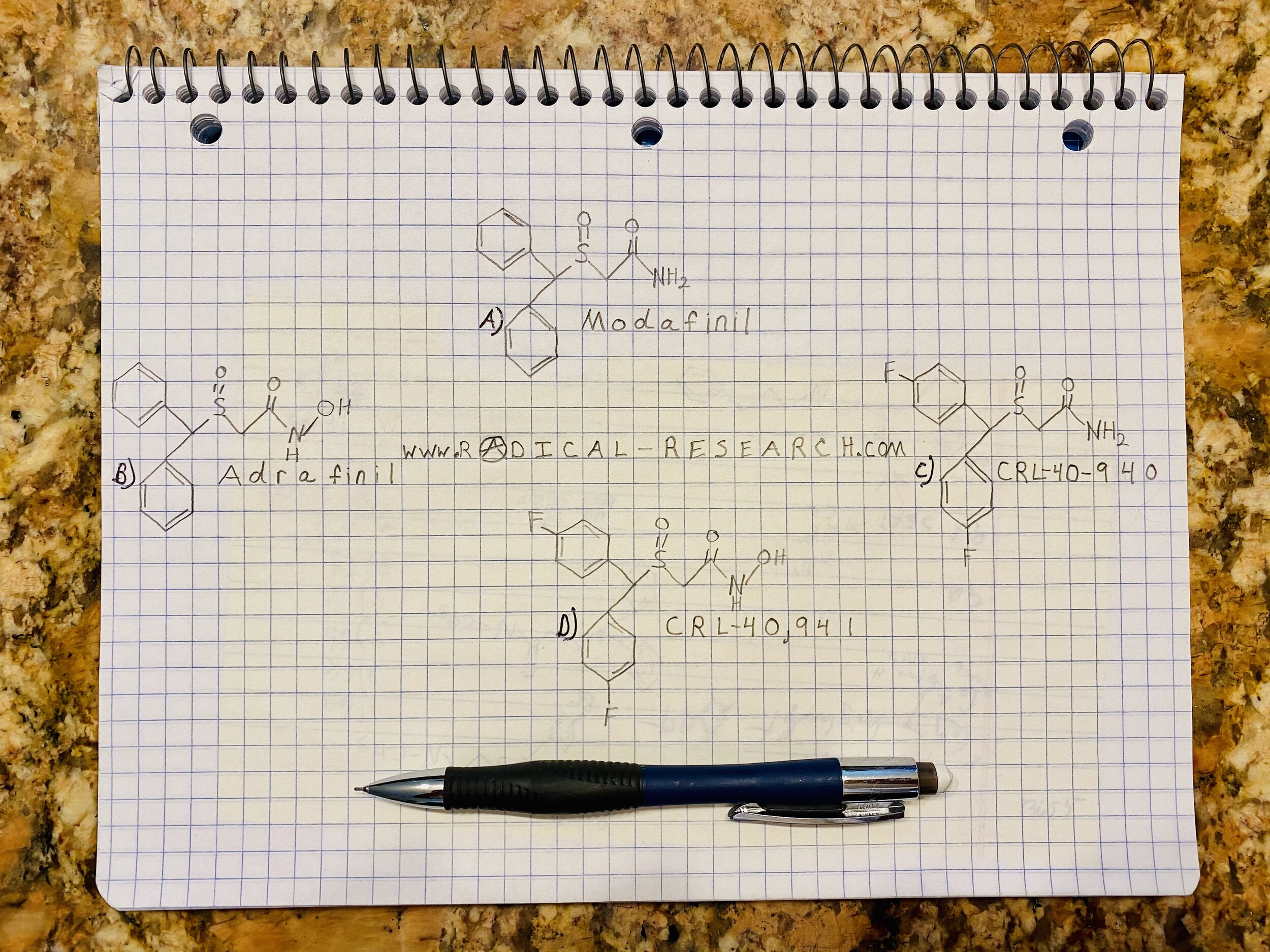
The interest in modafinil has led to the re-popularization of an old drug. This happens to be a precursor or prodrug to modafinil (Image A). The most prominent precursor is adrafinil (Image B). Adrafinil was developed as a pharmaceutical in France in the 1970s. It is now commonly sold as a nootropic or incorporated in more obscure nutritional supplement formulations. In vivo metabolism of adrafinil produces modafinil and modafinilic acid as main metabolites. CRL-40,941(Image D) is also referred to as fladrafinil and/or fluorafinil; this is simply adrafinil with fluorine halogenations at the 4,4 position. This compound should metabolize to Flmodafinil (Image C) just as adrafinil metabolizes to modafinil.
Comparison of modafinil, adrafinil, CRL-40-940, and CRL-40941 molecular structures.
Modafinil Structure Activity Relationship
Oluyomi (Yomi) Okunola-Bakare, Ph.D. and her colleagues published a tremendous paper in 2014 where they produced and evaluated many novel modafinil analogues to determine how modifications of the original structure would alter its function. The findings display an increased binding affinity at the DAT, SERT, and NET when the terminal amide carbonyl is removed. Analogues free of modified diphenyl rings and no amide carbonyl would generally increase DAT binding affinity with the addition of bulky substitutions on the terminal amine nitrogen. The analogues lacking the amide carbonyl displayed an increased binding affinity for DAT in the following order for these diphenyl ring para-position substitutions Br < Cl < F ≤ H. Conversely analogues retaining the amide carbonyl displayed increased DAT binding affinities in the following order H < F < Cl ≤ Br. These substitutions favored the DAT over SERT and NET. However, the addition of a 3-phenylpropyl group to the terminal amide enhanced binding affinities for all three transporters. The reduction of the double bonded oxygen to the sulfur resulted in decreased affinity for DAT but increased affinity for SERT.
Modafinil Analogues
As previously mentioned, researchers have produced many modafinil analogues to further understand the relationship between structure and function. The analogues have pharmacological profiles with unique monoaminergic interactions when compared to standard modafinil. These unique compounds can be selected to target specific indications. Researchers are in the beginning stages of drug development with modafinil analogues.
Cocaine Addiction Treatment Analogue
Perhaps the most interesting analogue is JJC8-016 (Image 1). This compound is bisflourinated, the terminal amide replaced by 3-phenolpropinate-substituted amine, and the terminal amide carbonyl was removed. This novel analog is a potential candidate for pharmacotherapeutic use in cocaine addiction. Currently there is no effective pharmaceutical treatment for this condition. This compound was chosen because its affinity for DAT and SERT were greater than those of R-MOD and similar those of cocaine. 20,21 JJC8-016 attenuated self-administration of cocaine and drug seeking behavior in rats. This compound also reduced self-administration of sucrose as well. A compound that can substitute for cocaine and protect against drug seeking behavior all while reducing sugar intake sounds great to me!
“Research Chem” Analogue
Modafiendz is an n-methylated bisflouro derivative of Modafinil which is commonly sold on recreational drug related, research chem sites. This compound has less binding affinity for the DAT than modafinil but shows mild affinity for SERT and NET whereas standard modafinil at a therapeutic dose is inactive at SERT and weak NET inhibitor .
Nootropic Analogue
The most common and accessible analogue is CRL-40,940 also known as; Flmodafinil, bisflouromodafinil, NLS-4, and lauflumide. This compound is simply modafinil which has had two fluorine molecules added at the 4 positions of the phenyl groups. This is referred to in the literature as para-halo-substituted which denotes “bisfluoro” in the name. The addition of these halogen groups produces an improved binding affinity for the DAT over standard modafinil. In mice this compound produces significantly longer wakefulness promoting periods than modafinil without significant sleep rebound. 22
Notable Research Analogues
Image 1: This JJC8-016 which was discussed above, this displayed the greatest affinity for the DAT. 20, 21
Image 2: Displays a removal of the oxygen molecule from both the terminal carbonyl and sulfoxide where they would typically be present in modafinil. This analogue was reported to have the greatest effect on inhibiting dopamine reuptake. 20
Image 3: This is the 4,4-dibrominated analogue of image 2. This compound was reported to be SERT selective and displayed nanomolar affinities at the SERT. 20
Image 4: The analogue with a 3,3 di-chloro halogenation and reduction of the sulfoxide was noted as being highly selective for DAT over SERT. 20
Image 5: The 4,4-dibromination along with the 3-phenylpropyl addition to modafinil produced an analogue with very similar binding affinities for SERT and DAT. 20
Modafinil analogues.
Modafinil Side Effects
Modafinil is generally tolerated well with the most common side effect being headache which was experienced by 17% of the population receiving the drug and 9% of the placebo group during the clinical trial. The percentage of those experiencing headache in the placebo group is an interesting observation to keep in mind when evaluating this side effect. The other common side effects that occurred in less than 8% of those receiving the drug were nausea, dizziness, and insomnia. The other side effects occurred in less than 5% of the experimental group.
The side effects are listed below with the percentage of those experiencing these side effects during the clinical trials. This has been transcribed directly from the Nuvigil prescribing info which is the insert that is provided to the consumer with the medication.1